- Arthropoda (Arthropods)
- Mantodea (Mantids)
-
Insecta

Fluorescence and color sensitivity

Like all scorpions Parabuthus villosus, too, fluoresces under UV light
The natural appearance of fluorescence is well known in several different groups of organisms like animals, fungi, bacteria, algae and plants. Like so, the jellyfish Aequorea victoria produces blue light through internal processes and then transduces it to green fluorescence by the protein GFP (Chalfie, 1995), whereas multiple species of reef fish show red fluorescence at their heads, eyes and fins (Michiels et al., 2008). In the class of the arachnids this phenomenon can be observed as well, a prominent example being the often brightly colored jumping spiders, of which for example Cosmophasis umbratica exhibits a gender specific green fluorescence, that only females develop (Lim et al., 2007). The fluorescence of scorpions is noteworthy because here this effect stretches without exception through all known recent species and is no local phenomenon, but is present on all sclerite surfaces of the cuticle with exception of the eyes and the tip of the aculeus. It is no wonder therefore, that the fluorescence of scorpions has been the target of much research and there have been many attempts to explaining its coming about and function.
In case of scorpions their fluorescence describes the emission of blue-green light under the exposure to ultraviolet light. It develops when the cuticle hardens, which is easily seen by the fact that freshly molted scorpions do not fluoresce at all and the fluorescence builds up from there, and is permanent even after death. It has been shown however, that the fluorescence of the cuticle can be greatly reduced by long exposure to high intensitiy ultraviolet light (Kloock, 2009).
The cuticle of the scorpion (Thicknesses in case of Hadrurus arizonensis) starting distally consists of epicuticle (0,3 µm), exocuticle (13 µm) and the endocuticle (65 - 85 µm) (Filshie and Hadley, 1979). All of those layers can be divided into sublayers, of which just the breaking down of the exocuticle into hyalin exocuticle (7,5 µm) and mesocuticle (5,5 µm) (Filshie and Hadley, 1979) is of interest to fluorescence (Hjelle, 1990).
The scorpions fluorescence has to date been ascribed to two different compounds in the hyaline cuticle, which are soluble in alcohol: beta-carboline (Stachel et al., 1999) and 4-methyl-7-hydroxycoumarin (Frost et al., 2001) - the latter of which being the first discovery of a coumarin compound in all the arthropods and the third in all the animals, usually coumarins are found in plants. It can be assumed, that due to its low concentration in the cuticle beta-carboline alone can not be responsible for the significant fluorescence of scorpions (Stachel et al., 1999).
Both compounds emit in the blue spectrum when exited by UV light, showing a maximum of intensity between 440 and 450 nm, which coincides with the emission maximum of 445 nm in case of an alcoholic solution made from the scorpions exuvia (Stachel et al., 1999; Frost et al., 2001), however falls short on the blue green emission of the real exuvia, which depending on species shows its maximum at around 500 nm (Kloock, 2008). Stachel et al., 1999, assume therefore that either the fluorescent agent in the solution differs from that in the exuvia itself, of local processes in the cuticle somehow skew the wavelength of the emitted light.
There are many hypothesis for the reason of this phenomenon, even though most have to face the problem that the recent scorpions are mostly nocturnal and the UV emission of the night sky is about 10000 fold lower than that of the sky during day (Johnsen et al., 2006). The hypotheses include (amongst others) the luring of prey, the discrimination of gender or species of other scorpions, a protective sunblock agains UV light and a sensory apparatus to detect UV light itself - however it is of course possible, that the fluorescence serves no purpose but is an entirely coincidental byproduct of the development of the cuticle. The first two hypotheses have already been refuted to some extent. It has been discovered, that aerial insects tend to avoid fluorescing scorpions (Kloock, 2005) and that due to the only very small differences in fluorescence in relation to gender, scorpions would have to be able to discriminate very fine differences in wavelength (2 nm), which seems unlikely (Kloock, 2008). Even though the intraspecific differences are a little bigger, this too seems unlikely on the same basis (Kloock, 2008).
To contemplate the hypothesis that the scorpions fluorescence serves as a UV sensor, one should relate this thought to the optical possibilities and the spectral discrimination of the scorpions eyes.
To investigate the spectral sensitivities of its optical senses physiological and behavioral studies have been performed. In one such study electrical impulses in the nervous system of the scorpion have been measured, that indicate, how many flashes of a certain wavelength are necessary to trigger a neuronal threshold response - the sensitivity is stated as the reciprocal of number of flahes by energy (E/i) (Machan, 1968). In the dark adapted state median and lateral eyes exhibit a siminar progression (leaving out absolute sensitivity) from 600 down to about 430 nm and significant differences below that mark (Machan, 1968). Where both eye types show a very low sensitivity at 600 nm, the sensitivity rises steadily to a maximum slightly over 500 nm and drops from there on until 430 nm (Machan, 1968). The sensitivity of the median eyes keeps on dropping and is vanishingly low at 370 nm, the sensitivity of the lateral eyes however rises drastically and assumes a maximum at 370 nm (Machan, 1968). The following graph shows these relative sensitivities, but no absolute values.
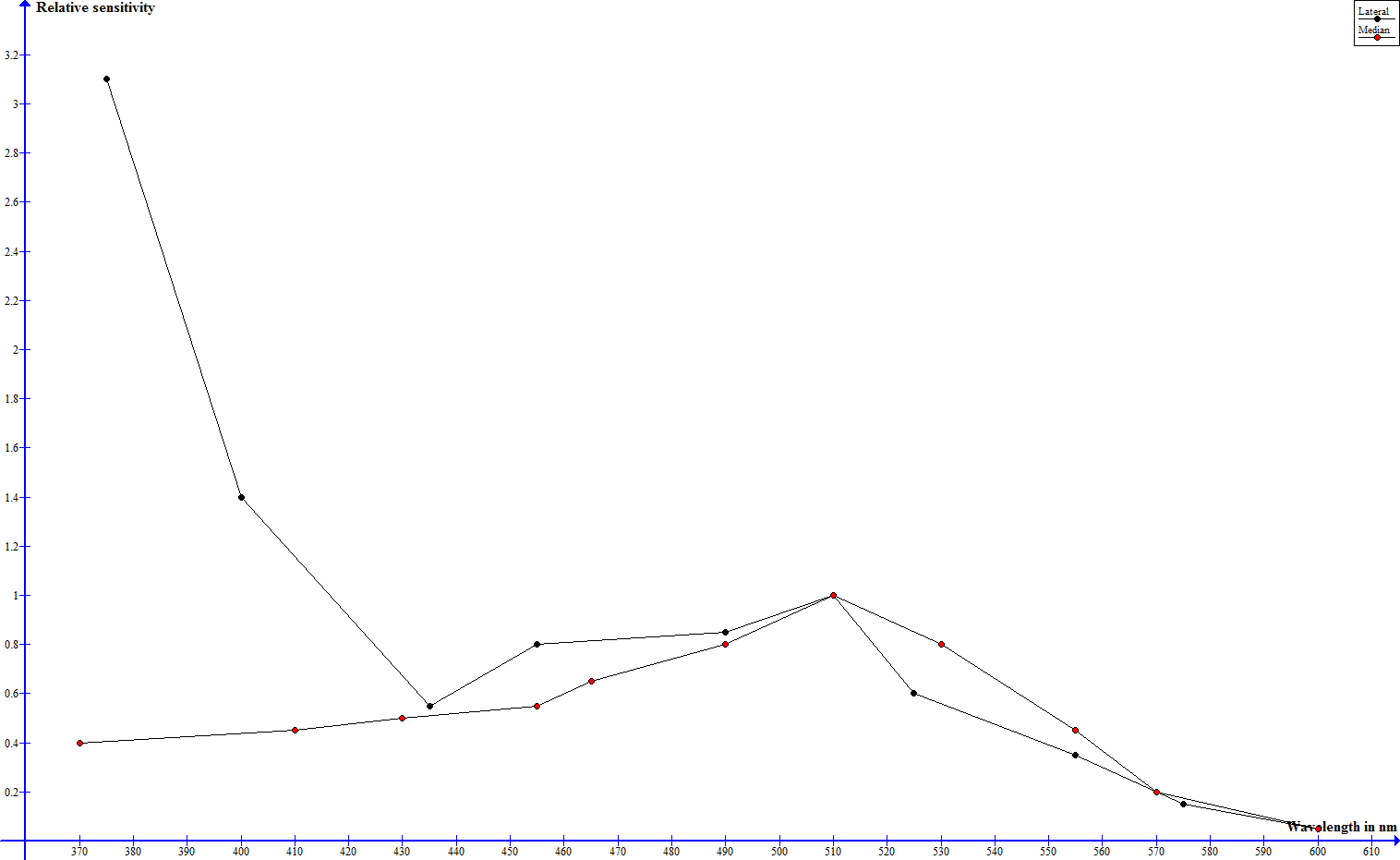
Spectral sensitivity of median and lateral eyes according to Machan, 1968, normalized to 1 at 509 nm - click to enlarge
Whereas the night adapted median eyes show their best perception in green wavelenghts, the lateral eyes are very sensitive towards green and to an extreme extent ultraviolet light. Yellow and red light are hardly perceived at all.
The great UV sensitivity of the lateral eyes drops higher-than-avarage when the eyes are light adapted, well underneath the sensitivity in the green range and the drop in sensitivity in the other spectral ranges is much smaller and more even, the median eyes exhibit an even drop throughout the hole spectrum (Machan, 1968). This can be explained by the absorption spectrum of the pigment granules in the median and lateral eyes. Whereas the absorpion spectrum is even in case of the median eyes, the absorption of the pigment granules in the lateral eyes is highest in the UV spectrum and drops steadily from there on towards higher wavelengths (Machan, 1968).
These findings are supported by behavioral experiments. Blass and Gaffin, 2007, researched the movement behaviors of scorpions when exposed to light of different wavelengths. They showed that when offered a retreat without light scorpions stayed the smallest amount of time in the UV exposed areas, followed by those exposed to green light. The animals spent most time in unsheltered areas when exposed to red or no light. In the same test series, but without offering a shelter, they observed that during the first 5 minutes of testing the scorpions exhibited a much higher movement frequency when exposed to UV light, again followed by green light and the lowest frequencies in case of red and no light. These experiments have been performed on not totally dark adapter specimen, so it comes to some surprise, that UV exposed specimen showed a higher aversion towards light and a higher movement frequency than those exposed to green light, since in those circumstances the eyes should have their highest sensitivity in the green spectrum. Since scorpions eyes just have one type of photo receptor (Brownell, 2001), but seem to be able to discriminate between different wavelengths of light, it can be assumed that they perform such discrimination by comparing intensities using different light sensitive organs, for example their extraocular light sense in the metasoma, which shows its highest sensitivity in the green spectrum (Rao and Rao, 1973).
To investigate whether the fluorescence can be used to detect ultraviolet light Kloock et al., 2010, exposed control specimen and those, whose fluorescence had been reduced, to light of different wavelengths and compared their behavior. The intesitity of the light had been chosen low enough to match that of the night sky, the human eye was not able to determine any fluorescence at all. It turned out that the reduction of fluorescence made no difference in case of infrared or infrared and white light combined. However, when exposed to infrared and ultraviolet light combined, the fluorecence reduced specimen showed a significantly increased activity, which indicates that scorpions do in fact use their fluorescence to detect ultraviolet light (Kloock et al., 2010).
Gaffin et al., 2012, performed a test series with a similar aim and monitored the movement frequency of unaltered scorpions and those who had their eyes covered under UV and green light. They observed a significantly lower activity when the eyes where covered, however the drop in activity was much higher in case of green light. It can therefore be argued, that even without the use of their eyes the animals were still able to detect enough UV light, which is evidence for the hypothesis that the fluorecent body serves as a UV collector, which transduces it to green light to then relay it to the nervous system via the extraocular light sense in the metasoma - this method would increase the uptake of UV light by four orders of magnitude compared to the uptake of UV light by the eyes alone due to the greatly broadened surface area (Gaffin et al., 2012).
References
Blass, G.R.C., Gaffin, D.D. (2008). Light wavelength biases of scorpions. Animal Behaviour 76, 365-373.
Brownell, P.H. (2001). Sensory ecology and orientational behaviors. In P.H. Brownell & G.A. Polis (ed.). Scorpion Biology and Research. Oxford University Press, Oxford.
Chalfie, M. (1995). Green fluorencet protein. Photochemistry and Photobiology 62, 651-656.
Frost, L.M., Butler, D.R., O’Dell, B. and Fet, V. (2001). A coumarin as a fluorescent compound in scorpion cuticle. In V. Fet and P.A. Selden (eds). Scorpions 2001 In Memoriam Gary A. Polis. British Arachnological Society, 363-368.
Filshie, B.K. und Hadley, N.F. (1979). Fine structures of the cuticule of the desert scorpion, Hadrurus arizonensis. Tissue and Cell 11(2), 248-262.
Gaffin, D.G., Bumm, L.A., Taylor, M.S., Popokina, N.V., Mann, S. (2012). Scorpion fluorescence and reaction to light. Animal Behaviour 83, 429-436.
Hjelle, J.T. (1990). Anatomy and morphology. In G.A. Polis (ed.). The biology of scorpions. Stanford University Press, Standford, California.
Johnsen S., Kelber A., Warrant E., Sweeney A.M., Widder E.A., Lee Jr. R.L., Hernández-Andrés J. (2006). Crepuscular and nocturnal illumination and its effect on color perception by the nocturnal hawkmoth Delilephila elpenor. The Journal of Experimental Biology 209, 789-800.
Kloock, C.T. (2005). Aerial insects avoid fluorescing scorpions. Euscorpius 21, 1–7.
Kloock, C.T. (2008). A comparison of fluorescence in two sympatric scorpion species. Journal of Photochemistry and Photobiology B: Biology 91, 132–136.
Kloock, C.T. (2009). Reducing scorpion fluorescence via prolonged exposure to ultraviolet light. The Journal of Arachnology 37, 368–370.
Kloock, C.T., Kubli, A., Reynolds, R. (2010). Ultraviolet light detection: a function of scorpion fluorescence. Journal of Arachnology 38, 441-445.
Lim, L.M.M., Land, M.F., Li, D. (2007). Sex-Specific UV and Fluorescence Signals in Jumping Spiders. Science 315, 481.
Machan, L. (1968). Spectral sensitivity of scorpion eyes and the possible role of shielding pigment effect. J. Exp. Biol. 49, 95-105.
Michiels, N., Anthes, N., Hart, N., Herler, J., Meixner, A., Schleifenbaum, F., Schulte, G., Siebeck, U., Sprenger, D., Wucherer, M. (2008). Red fluorescence in reef fish: A novel signalling mechanism?. BMC Ecology 8, 1635-1655.
Rao, G., Rao, K.P. (1973). A metasomatic neuronal photoreceptor in the scorpion. Journal of Experimental Biology 58, 189-196.
Stachel, S.J., Stockwell, S.A., Van Vranken, D.L. (1999). The fluorescence of scorpions and cataractogenesis. Chemistry & Biology 6, 531-539.
F. Schmitz, authored 2012